水木未来·视界iss.5丨借助冷冻电镜,蛋白质的呼吸机制首次被可视化

氧气和糖是动物、植物、真菌和许多细菌的生命之本。"呼吸"这一新陈代谢过程使得细胞能够将食物转化为能量。生物化学家Carola Hunte教授和她来自弗莱堡大学卓越中心CIBSS的团队借助冷冻电镜,以极高的精度首次可视化了那些为人类提供能量的蛋白质系统的结构和功能。该小组研究了由两个呼吸链复合物结合成的一组超复合物放线菌。
Oxygen and sugar are the basis of life for animals, plants, fungi and many bacteria. The metabolic process called respiration makes it possible to convert food into energy for the cells. Biochemist Prof. Dr. Carola Hunte and her team from the Cluster of Excellence CIBSS at the University of Freiburg have now visualized for the first time with unparalleled precision how an assembly of protein machines, which also supplies energy to humans, is structured and functions. The team studied two respiratory chain complexes fused into a supercomplex in a group of bacteria called Actinobacteria.
除了提供呼吸过程的基本阐释外,冷冻电镜的解析可以助力结核病或白喉治疗的新药研发。亨特解释说:"这些结构图像让我们体验了一次分子内部之旅,观察到他们的工作方式及一些奇特的现象。阐明结构的同时,也解释了超复合物是如何工作的。" 这项研究的结果发表在Nature Communications上,该研究是与法国国家科学研究中心的综合生物学中心(CBI)、遗传学和分子与细胞生物学研究所(IGBMC)的研究主任Bruno Klaholz博士、法国国家健康与医学研究院(Inserm)和法国斯特拉斯堡大学合作完成的。
In addition to providing a basic elucidation of respiratory processes, the cryogenic electron microscope analysis could aid in the development of new drugs to treat tuberculosis or diphtheria. "These images are like a journey into our molecular inner workings and its peculiar rules," Hunte explains, "Elucidating the structure simultaneously illuminates how the supercomplex works." The results of the study appeared in the journal Nature Communications and were produced in collaboration with Dr. Bruno Klaholz, research director at the Centre for Integrative Biology (CBI) / Institute of Genetics and of Molecular and Cellular Biology (IGBMC) of the CNRS, Inserm and the University of Strasbourg/France.
本文转载自Science Daily:
"Researchers visualize protein machinery of respiration for the first time"

细胞的能量货币
The energy currency of the cell
三磷酸腺苷(ATP)是细胞的能量货币——ATP由呼吸产生,并将能量从食物转移到细胞内以进行所有的细胞工作。受益于呼吸链的转化,二磷酸腺苷(ADP)被转化为富含能量的ATP。为了完成这一转化,呼吸链的蛋白质复合物在一个复杂的生化过程中用电子和质子形成跨越膜的电化学驱动力,该生化过程的动力由糖的燃烧提供。
Adenosine triphosphate (ATP) is the energy currency of the cell - the molecule is obtained during respiration and transfers energy from food to all processes in the cell. Thanks to the processes on the respiratory chain, adenosine diphosphate is turned into the energy-rich ATP. To do this, protein complexes of the respiratory chain build up an electrochemical driving force across a membrane with electrons and protons in a complicated chemical-physical process that is powered by the combustion of sugar.
该研究的第一作者Hunte团队的Wei-Chun Kao博士解释说:"我们分析了呼吸系统细胞色素bcc-aa3超复合物,二十六个蛋白质组成了这个蛋白质系统。在这之前,我们并不清楚他们的分子力和动力的具体互动。" 研究人员发现,该复合物的质子泵与人类非常相似,但电子被电子载体醌接管的部分在细菌中显示出明显的差异。"这个差异使我们可以借机开发特定的药剂,通过干扰呼吸链来杀死致病放线菌,如结核分枝杆菌或白喉杆菌,"Hunte补充说。
"We analyzed the respiratory cytochrome bcc-aa3 supercomplex. Twenty-six proteins make up the protein machine. The exact interaction of molecular forces and dynamics is not well understood yet, and this is where such a detailed description helps us," explains the study's first author Dr. Wei-Chun Kao of Hunte's team. The proton pump of the complex is very similar to humans, the researchers find, but the part where electrons are taken over by the electron carrier quinone shows clear differences in the bacterium. "This is where we could tie in and develop specific agents that kill pathogenic actinobacteria such as Mycobacterium tuberculosis or Corynebacterium diphtheriae by interfering with the respiratory chain," Hunte adds.

Image@ Wei-Chun Kao/CIBSS/University of Freiburg

具有原子分辨率的冷冻电镜
Cryogenic microscope with atomic resolution
冷冻电镜(Cryo-EM)是一种在零下183摄氏度的低温下用高分辨率显微镜检查样品的技术,可以将结构解析到单原子水平。在这个过程中,机器学习算法被用来进一步完善收集到的数据。
Cryogenic electron microscopy (Cryo-EM) is a technique that examines samples at low temperatures of - 183 Celsius in a high-resolution microscope and can resolve structures to the level of single atoms. In the process, machine learning algorithms are used to further refine the collected data.
"有了这些(冷冻电镜)数据,我们可以更好地了解新陈代谢和信号传导的相互作用,这是CIBSS卓越中心目前的重点课题。"—Carola Hunte教授,弗莱堡大学CIBSS卓越中心
“With this data, we can also better understand the interplay of metabolism and signaling, which is a particular focus in the Cluster of Excellence CIBSS."—Prof. Dr. Carola Hunte, Cluster of Excellence CIBSS, University of Freiburg


支持性内容
Supportive content
相关Nature Communications文章:
"Structural basis for safe and efficient energy conversion in a respiratory super-complex", DOI: 10.1038/s41467-022-28179-x
学界认为,质子转运呼吸复合物组成超复合物的举动是为了提高能量转换的效率,限制有氧细胞呼吸过程中有害活性氧的产生。细胞色素bc复合体和细胞色素aa3氧化酶是质子动力的主要驱动力,通过呼吸作用为ATP的产生提供动力,但在超复合物的状态下,用何种方法转移消耗掉的电子和质子以提高安全性和效率还不清楚。
Proton-translocating respiratory complexes assemble into supercomplexes that are proposed to increase the efficiency of energy conversion and limit the production of harmful reactive oxygen species during aerobic cellular respiration. Cytochrome bc complexes and cytochrome aa3 oxidases are major drivers of the proton motive force that fuels ATP generation via respiration, but how wasteful electron- and proton transfer is controlled to enhance safety and efficiency in the context of supercomplexes is not known.
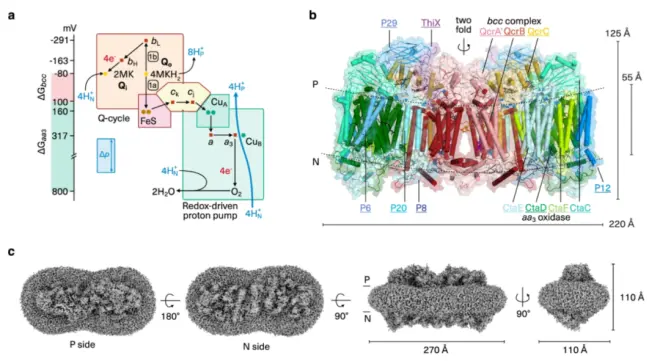
A. 呼吸体蛋白超复合物中能量转换的示意图。细胞基数交换复合物在Qo处与甲基萘酚氧化一起运行Q循环,与质子释放到正电膜侧的位点耦合(H+P),并在 Q 时减少甲萘醌我从电负性膜侧吸收质子的位点(H+N),通过分叉电子转移链接。氧化酶作为氧化还原驱动的质子泵运行。
A. Schematic presentation of energy conversion in the obligate respiratory supercomplex. The cyt bcc complex operates a Q cycle with menaquinol oxidation at the Qo site coupled to proton release to the electropositive membrane side (H+P), and menaquinone reduction at the Qi site with proton uptake from the electronegative membrane side (H+N), linked through bifurcated electron transfer. The oxidase operates as a redox-driven proton pump.
B. 细胞性密闭锁细胞的冷冻电镜结构3超级复杂。同源二聚体的原子模型平行于透明表面显示的膜且叠加。亚基采用颜色编码,并带有匹配的下划线标签。
B. Cryo-EM structure of cyt bcc-aa3 supercomplex. The atomic model of the homodimer is viewed parallel to the membrane shown in transparent surface and superimposed in cartoon representation. Subunits are colour-coded with matching underlined labels.
C. 具有去垢剂的超复合物的3D重建。P和N分别表示膜的正极和负极。实验图的等值线水平设置为3.5均方根偏差(rmsd)。
C. 3D reconstruction of supercomplex with dimensions and detergent micelle. P and N denote the electro-positive and -negative sides of the membrane, respectively. The contour level of the experimental map was set to 3.5 root mean square deviation (rmsd).
我们通过解析谷氨酸放线菌的细胞色素bcc-aa3(III2-IV2)超复合物的2.8 Å分辨率冷冻电镜结构,解决了甲萘醌、底物模拟物、番茄红素、意外的Qc位点、分子氧、质子转移路线以及关键质子化残基的构象状态等问题。我们的研究结果阐释了在呼吸体蛋白超复合物中,如何通过控制电子和质子转移实现安全和高效的能量转换。该冷冻电镜结构可以指导引起白喉和肺结核的放线菌的针对性药物的合理设计。
Here, we address this question with the 2.8 Å resolution cryo-EM structure of the cytochrome bcc-aa3 (III2-IV2) supercomplex from the actinobacterium Coryne-bacterium glutamicum. Menaquinone, substrate mimics, lycopene, an unexpected Qc site, dioxygen, proton transfer routes, and conformational states of key protonable residues are resolved. Our results show how safe and efficient energy conversion is achieved in a respiratory supercomplex through controlled electron and proton transfer. The structure may guide the rational design of drugs against actinobacteria that cause diphtheria and tuberculosis.
水木视界丨iss. 9

每周分享结构生物学前沿进展


